
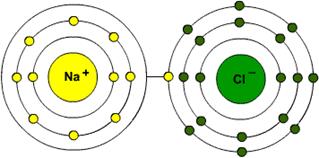
Iron (Fe), for example, can form both an iron ion with 2+ (Fe 2+) and an iron ion with 3+ (Fe 3+).Ī neutral sodium (Na) atom loses one electron to form a sodium ion (Na +) with a charge of 1+ (see Table 2.8). Some elements can form ions with two or more different charges. All the remaining metal elements produce at least one ion with a charge of 2+.Group 16 elements gain two electrons to become 2–, and Group 15 elements gain three electrons to become 3–. They can gain one electron and become 1–. Nonmetals in Group 17 need just one electron to complete their valence shell.Group 2 elements give up two electrons to become 2+, and Group 3 give up three electrons to become 3+. They can give up this one electron and become 1+. Metals in Group 1 have only one electron in their valence shell.In general, atoms form ions according to the following patterns:
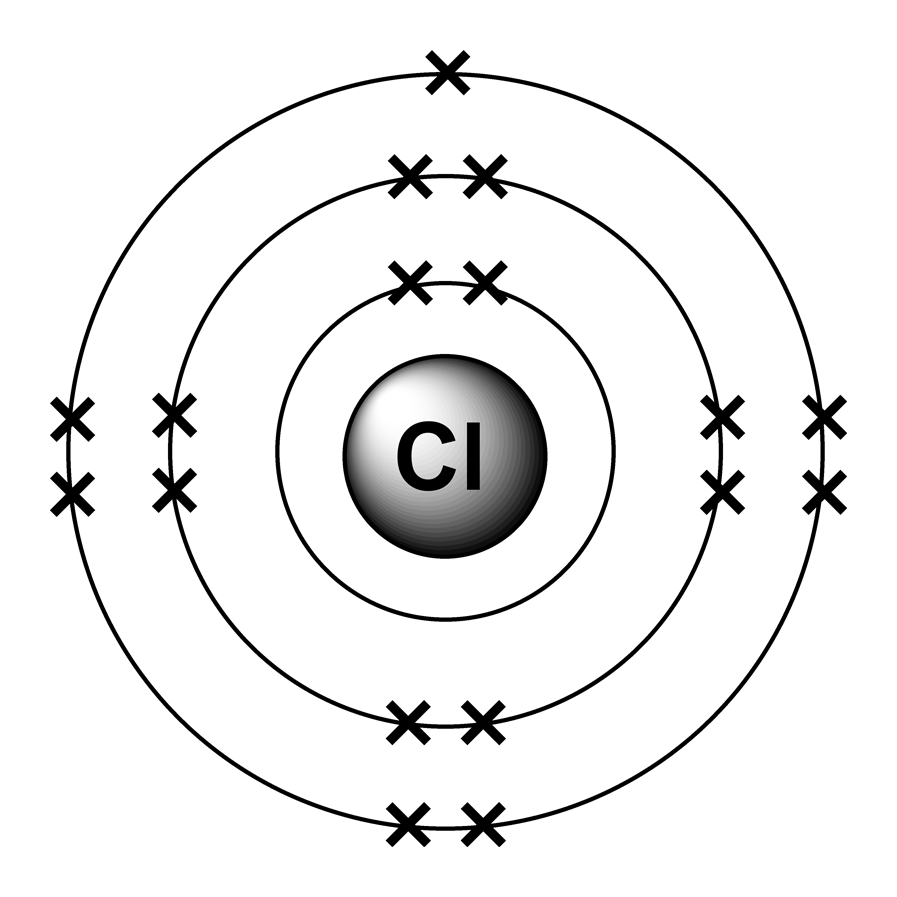
#ELEMENT CL PROTONS FULL#
In other words, if an atom could lose one electron or gain seven to have a full octet, it will lose one. Atoms tend to gain or lose the least number of electrons to achieve a full octet. Elements will gain or lose electrons to have the same configuration as a noble gas, in other words, to have a full octet. The periodic table can help in predicting the type of ion that an element will form based on how many electrons need to be gained or lost for it to become stable. 2.22 B, sodium lost one electron so that it had eight electrons in shell 2, which becomes its valence shell. 2.22 A, the oxygen atom gained two electrons so that it had eight electrons in shell 2, its valence shell. Other elements will gain or lose electrons to achieve completely full valence shells, eight electrons in the valence shell, so that they are also stable. The noble gases have eight electrons in their valence shells. The octet rule states that regardless of how many electrons a shell can potentially hold, the valence shell can only hold eight electrons. This is because they have completely full valence electron shells. Group 18 elements, the noble gases, are very stable (non-reactive). This gives it one less negative charge than positive charges and an overall charge of 1+. 2.22 B, a neutral sodium atom (Na), with 11 protons and 11 electrons, loses one electron. Metal elements tend to lose electrons and become cations. This gives it two more negative charges than positive charges and an overall charge of 2–. 2.22 A, a neutral oxygen atom (O), with eight protons and eight electrons, gains two electrons. Nonmetals tend to gain electrons and become anions. A positive ion or cation is an atom that has lost electrons. An atom that has lost negatively charged electrons becomes positive. A negative ion or anion is an atom that has gained electrons. An atom that has gained negatively charged electrons becomes negative. Therefore, the atom is no longer neutral.
When an atom gains or loses an electron, the atom no longer has a balanced charge. While the atomic number, the number of protons in the nucleus, never changes, some electrons are easily lost or gained by an atom. On the other hand, electrons that have only one electron in their valence shells (Group 1 elements) or elements that are just one electron short of having a complete shell (Group 17) are the most reactive. For example, atoms with complete valence shells, the noble gases, are the least chemically reactive. In general, the electrons in valence shells determine how the atom behaves in chemical reactions. The valence shell is the outermost electron shell of an atom. In other words, the farther the shell is from the nucleus, the larger it is, and the higher its average energy. 2.21 C).Īs the electron shells go from 1 to 7, they increase in size and average energy.


 0 kommentar(er)
0 kommentar(er)
